The National Agency for Food and Drug Administration and Control (NAFDAC) has approved AstraZeneca COVID-19 vaccine for use in Nigeria. This information was made public on Thursday 18 February 2021 by Bashir Ahmad, the Personal Assistant on New Media to President Muhammadu Buhari.
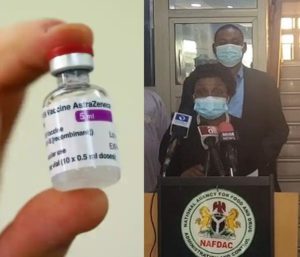
During the press conference, Director-General of NAFDAC, Professor Mojisola Adeyeye said the agency received the dossier of the vaccine a week ago from Serum Institute of India on February 10, 2021.
The Oxford/AstraZeneca vaccine, also known as ChAdOx1 nCoV-19, or AZD1222, is a viral vector vaccine.
Scientists used an adenovirus, originally derived from chimpanzees, and modified it to train the immune system to mount a strong response against SARS-CoV-2 (the virus that causes COVID-19).
COVISHIELD was found safe and well-tolerated in adults above 18 years of age.
Prof. Adeyeye said the vaccine can be stored at 2 to 8-degree centigrade. However, this is a major issue as the complex logistics of managing the vaccine cold chain, combined with the limited government funding may aggravate the logistical challenge of transporting and distributing those vaccines quickly in the country.
According to the DG, there are three additional vaccines undergoing evaluation, but the evaluation on Astrazeneca shows that the vaccine is effective against the UK variant of the virus which has been reported in Nigeria.
She further disclosed that the South African variant has not been reported in Nigeria and that the agency has over 30 herbal medicine undergoing review for listing.
South Africa suspended the use of the Oxford/AstraZeneca vaccine earlier this month after it found out the vaccine could not prevent mild and moderate illness caused by a variant found in the country. The country announced on Tuesday that it would donate the jabs already procured to the African Union.
“We don’t yet know how well this new variant will spread, but if it is successful it can be presumed that immunity from any vaccine or previous infection will be blunted,” Dr Simon Clarke, an associate professor of cellular microbiology at the University of Reading told the media.
“I think that until we know more about these variants, any variants which carry E484K should be subject to surge testing as it seems to confer resistance to immunity, however that is generated.”
However, the Nigerian government is looking to vaccinate a large percentage of its about 200 million population this year.
The African biggest economy has missed out on the first phase of the Pfizer COVID-19 vaccine, as WHO claimed the country doesn’t have adequate facility to store the vaccine.
The 100,000 doses of the Pfizer vaccine initially expected in the country were replaced with 16 million doses of Astrazeneca vaccine.
Nigeria which has to date registered 149,000 Coronavirus cases and 1,787 deaths is expected to get the first batch of the COVID_19 vaccines by the end of February.